Continue boiling it until the water is gone and youre left with the salt. The mixture is poured into the filter paper.

Separating Mixtures Science Poster Chemistry Classroom Chemistry Science Poster
- Then after that turn of the burner and allow the heated solution to cool off a bit.

. The structure and nature of this crystalline network will depend on the conditions under which. Evaporating Evaporation Separating a soluble. To Separate a Dissolved Solid from a Solution.
Filtration and Crystallization Chemistry 10th Grade. Warm the solution by placing the evaporating basin over a boiling water bath. In the process of crystallization A solid is formed with atoms or molecules in organized structures which are called crystalline networks.
110 describe these experimental techniques for the separation of mixtures. Filtration separates solids of different sizes. Filtering sand and water mixture.
Describe how you would separate rock salt to obtain salt crystals and pure dry sand. Crystallization is a separation process that makes use of differences in solubility of the components present in the melt or the solution. Crystallisation is a separation technique that is used to separate.
The sand does not pass through and is left behind residue but the water passes through the filter paper and is collected in the conical flask filtrate. To separate a mixture of two or more miscible liquids for which the difference in boiling points is less than 25 K fractional distillation process is used for example for the separation of different gases from air different fractions from petroleum products etc. So let us find out if that is the case.
A beaker containing a mixture of insoluble solid and liquid. Crystallization is generally used to obtain the pure form or crystal of any substance. Spelling grammar organising your ideas and information clearly and using key scientific words.
In this question you get marks for how well your answer is written. Let the water drain and leave the insoluble solid to dry. You will get marks for.
Crystal the word makes us think of a solid shiny object which may be rare or precious. Chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points.
Filter paper is ideal to filter off sand from water. There is filter paper in a filter funnel above another beaker. This process is carried out by cooling highly concentrated solutions.
It leads to Physical Changes in a Material. To obtain large regularly shaped crystals. Crystallization is a powerful and versatile technique to separate components from a liquid mixture.
As it can possibly contain is called a saturated solution. Evaporation is used to separate a soluble solid from a liquid. Eg- Water and sand mixture.
Pour the mixture through the filter funnel. Involves separating mixtures based on the different boiling points of the liquids in a solution. Crystallisation is the process of formation of crystals.
To Separate an Undissolved Solid from a Mixture of the Solid and a Liquid Solution. Evaporation removes a liquid from a solution to leave a solid material. Put the solution in an evaporating basin.
When water is evaporated from a mixture to form substance crystals Distillation. Now the process involved in this separation is as follows. Mixtures can be separated using a variety of techniques.
Put the solution in an evaporating basin. 5 IIii Describe how you would obtain a pure sample of copper sulfate-5-water crystals from a mixture of copperII sulfate-5-water with copper oxide using some of the techniques listed above. The solution is warmed in an open container allowing the solvent to evaporate leaving a saturated solution.
We can separate mixtures of water and an insoluble substance like sand by filtering. Pour the salt water back into the empty pan. This liquid mixture may be an impure melt or a solution which refers to the case that one or more components are dissolved in a liquid solvent.
A solution that has as much solid dissolved in it. This lesson plan includes the objectives prerequisites and exclusions of the lesson teaching students how to separate mixtures using filtration and crystallization decide the apparatus needed and determine when each should be used. - Add water to the mixture in the container.
Simple distillation fractional distillation filtration crystallisation paper chromatography. Another way you can separate the salt water and sand is to stir up the sandsalt water and pour it through a coffee filter to capture the sand. Warm the solution by placing the evaporating basin over a boiling water bath.
Crystals and crystalline networks can be formed through the precipitation of a solution by fusion and in some cases by direct deposition of a gas. Stop heating before all the solvent has evaporated. Heat the salt water until the water boils.
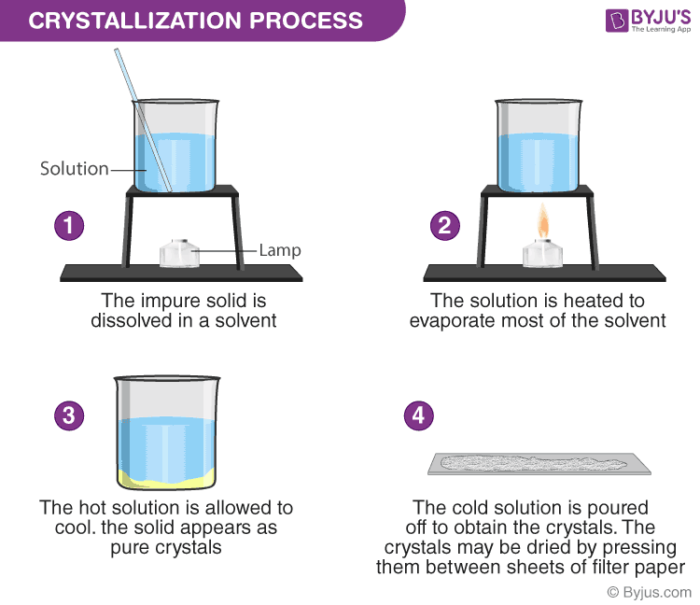
Crystallization is the process of separating a solid from a liquid solution. Stop heating when crystals begin to form around the edge of. This method is used to obtain a salt which contains water of crystallisation from a salt solution.
- Pour the mixture into a container. A mixture of two amino acids glycine and alanine. A solid that has dissolved in a liquid and made a solution.
As the saturated solution is allowed to cool. - Turn on a burner and heat this solution in the container till the salt has dissolved completely. Rock salt is a mixture of salt sodium chloride and sand.
Separating sand from Salt water. Add HCl to react with the carbonate. The products are zinc chloride CO2 and.
This process is called crystallisationThe crystallisation method is used. Describe how you would obtain pure crystals of Sodium chloride from a muxture of solid sodium chloride and solid zinc carbonate Step1 Step 2 Step 3 1.
Crystallization Definition Process Separation Technique Faqs

Chemistry Science Mixtures Separation Lesson Activities Chemistry Lessons Lessons Activities Chemistry

Mixtures And Separating Mixtures Separating Mixtures Fractional Distillation Paper Chromatography

0 Comments